LINK DOWNLOAD : LEUKOPOIESIS
Tuesday, September 11, 2012
LINK DOWNLOAD : LEUKOPOIESIS
LEUKOPOIESIS
Granulopoiesis
The maturation process of granulocytes takes place with cytoplasmic changes characterized by the synthesis of a number of proteins that are packed in two organelles: the azurophilic and specific granules. These proteins are produced in the rough endoplasmic reticulum and the Golgi complex in two successive stages. The first stage results in the production of the azurophilic granules, which stain with basic dyes in the Wright or Giemsa methods and contain enzymes of the lysosomal system. In the second stage, a change in synthetic activity takes place with the production of several proteins that are packed in the specific granules. These granules contain different proteins in each of the three types of granulocytes and are utilized for the various activities of each type of granulocyte.
Maturation of Granulocytes
The myeloblast is the most immature recognizable cell in the myeloid series. It has a finely dispersed chromatin, and nucleoli can be seen. In the next stage, the promyelocyte is characterized by its basophilic cytoplasm and azurophilic granules. These granules contain lysosomal enzymes and myeloperoxidase. The promyelocyte gives rise to the three known types of granulocyte. The first sign of differentiation appears in the myelocytes, in which specific granules gradually increase in quantity and eventually occupy most of the cytoplasm. These neutrophilic, basophilic, and eosinophilic myelocytes mature with further condensation of the nucleus and a considerable increase in their specific granule content. Before its complete maturation, the neutrophilic granulocyte passes through an intermediate stage in which its nucleus has the form of a curved rod (band cell).
Maturation of Lymphocytes & Monocytes
Study of the precursor cells of lymphocytes and monocytes is difficult, because these cells do not contain specific cytoplasmic granules or nuclear lobulation, both of which facilitate the distinction between young and mature forms of granulocytes. Lymphocytes and monocytes are distinguished mainly on the basis of size, chromatin structure, and the presence of nucleoli in smear preparations. As lymphocyte cells mature, their chromatin becomes more compact, nucleoli become less visible, and the cells decrease in size. In addition, subsets of the lymphocyte series acquire distinctive cell-surface receptors during differentiation that can be detected by immunocytochemical techniques.
Lymphocytes
Circulating lymphocytes originate mainly in the thymus and the peripheral lymphoid organs (eg, spleen, lymph nodes, tonsils). However, all lymphocyte progenitor cells originate in the bone marrow. Some of these lymphocytes migrate to the thymus, where they acquire the full attributes of T lymphocytes. Subsequently, T lymphocytes populate specific regions of peripheral lymphoid organs. Other bone marrow lymphocytes differentiate into B lymphocytes in the bone marrow and then migrate to peripheral lymphoid organs, where they inhabit and multiply in their own special compartments.
The first identifiable progenitor of lymphoid cells is the lymphoblast, a large cell and dividing two or three times to form prolymphocytes. Prolymphocytes are smaller and have relatively more condensed chromatin but none of the cell-surface antigens that mark prolymphocytes as T or B lymphocytes. In the bone marrow and in the thymus, these cells synthesize cell-surface receptors characteristic of their lineage, but they are not recognizable as distinct B or T lymphocytes in routine histological procedures. Using immunocytochemical techniques makes the distinction.
Monocytes
The monoblast is a committed progenitor cell that is almost identical to the myeloblast in its morphological characteristics. Further differentiation leads to the promonocyte, a large cell (up to 18 um in diameter) with a basophilic cytoplasm and a large, slightly indented nucleus. The chromatin is lacy, and nucleoli are evident. Promonocytes divide twice in the course of their development into monocytes. A large amount of rough endoplasmic reticulum is present, as is an extensive Golgi complex in which granule condensation can be seen to be taking place. These granules are primary lysosomes, which are observed as fine azurophilic granules in blood monocytes. Mature monocytes enter the bloodstream, circulate for about 8 h, and then enter the connective tissues, where they mature into macrophages and function for several months.
source : Basic Histology
Posted in 2012 , hematology , materi kuliah
Friday, July 20, 2012
Cara Menulis Summary Jurnal (How to Write a Summary)
- Write in the present tense.
- Make sure to include the author and title of the work.
- Be concise: a summary should not be equal in length to the original text.
- If you must use the words of the author, cite them.
- Don't put your own opinions, ideas, or interpretations into the summary. The purpose of writing a summary is to accurately represent what the author wanted to say, not to provide a critique.
Wednesday, July 11, 2012
Pengantar Praktikum Female Genital Histology
- Understand and identify the stages of follicular growth (primordial, primary, secondary, tertiary), as well as the changes that occur in the follicular wall during pregnancy.
- Identify the structure of the oviduct.
- Describe the changes that occur in endometrium during the menstrual cycle as well as the changes that occur during pregnancy.
- Identify the structure of the vagina
Anda Bisa Download Versi PDF di alamat ini : PENGANTAR PRAKTIKUM FEMALE GENITAL HISTOLOGY
Posted in 2012 , female reproductive system , praktikum
Pengantar Praktikum Male Genital Histology
- Recognize germ cells at different steps of spermatogenesis in the seminiferous tubule.
- Recognize Sertoli cells and Leydig cells, and explain their roles in the production of sperm and regulation of the male reproductive system.
- Recognize the various parts of the male reproductive tract in histological section, and explain the contribution of each part to the production of semen for the final ejaculate.
- Recognize and understand the histological organization of the prostate gland, Seminal vesicle gland dan Bulbourethral Glands
TESTIS
| TIPS MEMBEDAKAN SEL SERTOLI, SPERMATOGONIUM DAN SPERMATOSIT PRIMER |
| Sel Sertoli menyelimuti sel-sel gamet selama proses maturasinya. Sel Sertoli dapat dibedakan karena mereka memiliki warna pucat dengan nukleus berbentuk oval inti dan bentuk sel yang tidak beraturan, batas antar sel tidak jelas Inti |
| Spermatogonia adalah sel-sel prekursor yang besar dan selalu terletak di sepanjang membran basal tubulus. |
| Spermatosit primer memiliki ukuran paling besar (diantara sel-sel gamet), inti heterochromatic, dan mereka terletak di antara membran basal dan lumen tubulus. |
SLIDE M3
Pada slide ini selain bisa mengamati parenkim testis yang dibungkus tunika albugenia, Anda juga bisa mengamati mediastinum testis dengan rete testis didalamnya, ductus epididymis dan awal dari ductus deferents.
Dengan pembesaran kecil coba identifikasi testis yang dibungkus tunika albugenia dengan tubulus seminiferus didalamnya. Coba ingat kembali perjalanan spermatozoa setelah dilepas dari tubulus seminiferus, ductus apakah yang dilewati hingga spermatozoa mencapai epididymis dimana spermatozoa akan disimpan sampai saatnya ejakulasi?
Coba geser pengamatan Anda sepanjang tunika albugenia dan temukan bagian tunika albugenia yang paling tebal, bagian ini disebut mediastinum. Didalam mediastinum coba identifikasi saluran-saluran dengan bentuk tidak teratur yang disebut sebagai rete testis. Ductus apakah yang dilewati spermatozoa sebelum mencapai rete testis ??? dan bagaimanakan strukturnya ???
Amati bahwa rete testis dilapisi oleh sel epitel kuboid dan Anda mungkin melihat mikrovili dibagian atas epitel.
SLIDE M2
Pada slide ini bisa diamati epididymis, Anda bisa mengamati epididymis yang berupa tabung dengan spermatozoa dilumennya. Coba identifikasi epitel yang melapisi dinding epididymis dan identifikasi otot polos pada dindingnya !!!
SLIDE M5
Posted in 2012 , male genital system , praktikum
Monday, July 2, 2012
Sperm transport in the female reproductive tract
source : http://herkules.oulu.fi/isbn9514266641/html/x480.html
Sperm maturation and transport in the male reproductive tract
Wednesday, June 27, 2012
The Histology of Female Genital System Semester VI (2012)
Female Reproductive System
Ovaries
Ovarian Follicles
Uterus
Menstrual Cycle
Mammary Gland
Female Reproductive System
 The female reproductive system consists of the internal reproductive organs (the paired ovaries and oviducts, the uterus, and the vagina) and the external genitalia.
The female reproductive system consists of the internal reproductive organs (the paired ovaries and oviducts, the uterus, and the vagina) and the external genitalia.
Primary Follicle

Secondary Follicle
 Secondary follicles are similar to primary follicles except for the presence of accumulations of liquor folliculi among the granulosa cells.
Secondary follicles are similar to primary follicles except for the presence of accumulations of liquor folliculi among the granulosa cells.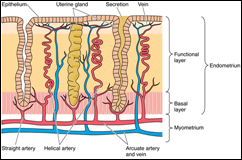 The uterus, a single, thick, pear-shaped structure located in the midline of the pelvis, receives at its broad, closed end the terminals of the paired oviducts. It is divided into three regions, the body, fundus, and the cervix.
The uterus, a single, thick, pear-shaped structure located in the midline of the pelvis, receives at its broad, closed end the terminals of the paired oviducts. It is divided into three regions, the body, fundus, and the cervix.Mammary Gland
- Color Textbook Histology, third edition, leslie P. Gartner
- Basic Histology, tenth edition, L. Carlos Junqueira,2003
- Histology and Cell Biology, second edition,2007
- Elsevier’s Integrated Histology,2007
Posted in 2012 , genital system , lecture , materi kuliah , reproductive system
Histology of Male Genital System Semester VI (2012)
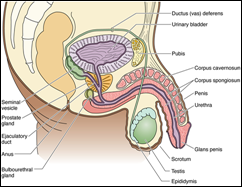 The male reproductive (genital) system consists of the two testes suspended in the scrotum, a system of intratesticular and extratesticular genital ducts, associated glands, and the male copulatory organ, the penis. The testes are responsible for the formation of the male gametes, known as spermatozoa, as well as for the synthesis, storage, and release of the male sex hormone, testosterone.
The male reproductive (genital) system consists of the two testes suspended in the scrotum, a system of intratesticular and extratesticular genital ducts, associated glands, and the male copulatory organ, the penis. The testes are responsible for the formation of the male gametes, known as spermatozoa, as well as for the synthesis, storage, and release of the male sex hormone, testosterone.TESTIS
 Each testis is surrounded by a capsule known as the tunica albuginea. Immediately deep to this layer is a highly vascularized loose connective tissue, the tunica vasculosa. The posterior aspect of the tunica albuginea is somewhat thickened, forming the mediastinum testis, from which connective tissue septa radiate to subdivide each testis into approximately 250 compartments known as the lobuli testis
Each testis is surrounded by a capsule known as the tunica albuginea. Immediately deep to this layer is a highly vascularized loose connective tissue, the tunica vasculosa. The posterior aspect of the tunica albuginea is somewhat thickened, forming the mediastinum testis, from which connective tissue septa radiate to subdivide each testis into approximately 250 compartments known as the lobuli testis The wall of the seminiferous tubule is composed of a slender connective tissue layer, the tunica propria, and a thick seminiferous epithelium, separated from each other by a well-developed basal lamina.
The wall of the seminiferous tubule is composed of a slender connective tissue layer, the tunica propria, and a thick seminiferous epithelium, separated from each other by a well-developed basal lamina.SPERMATOZOON
 Spermatids discard much of their cytoplasm and form a flagellum to become transformed into spermatozoa, a process known as spermiogenesis.
Spermatids discard much of their cytoplasm and form a flagellum to become transformed into spermatozoa, a process known as spermiogenesis. The intratesticular genital ducts are the tubuli recti (straight tubules), the rete testis, and the ductuli efferentes. These ducts carry spermatozoa and liquid from the seminiferous tubules to the ductus epididymidis.
The intratesticular genital ducts are the tubuli recti (straight tubules), the rete testis, and the ductuli efferentes. These ducts carry spermatozoa and liquid from the seminiferous tubules to the ductus epididymidis. The ductus epididymidis is a single highly coiled tube about 4-6 m in length. Together with surrounding connective tissue and blood vessels, this long canal forms the body and tail of the epididymis. It is lined with pseudostratified columnar epithelium composed of rounded basal cells and columnar cells. These cells are supported on a basal lamina surrounded by smooth muscle cells, whose peristaltic contractions help to move the sperm along the duct, and by loose connective tissue rich in blood capillaries. Their surface is covered by long, branched, irregular microvilli called stereocilia. The epithelium of the ductus epididymidis participates in the uptake and digestion of residual bodies that are eliminated during spermatogenesis.
The ductus epididymidis is a single highly coiled tube about 4-6 m in length. Together with surrounding connective tissue and blood vessels, this long canal forms the body and tail of the epididymis. It is lined with pseudostratified columnar epithelium composed of rounded basal cells and columnar cells. These cells are supported on a basal lamina surrounded by smooth muscle cells, whose peristaltic contractions help to move the sperm along the duct, and by loose connective tissue rich in blood capillaries. Their surface is covered by long, branched, irregular microvilli called stereocilia. The epithelium of the ductus epididymidis participates in the uptake and digestion of residual bodies that are eliminated during spermatogenesis. From the epididymis the ductus (vas) deferens, a straight tube with a thick, muscular wall, continues toward the prostatic urethra and empties into it. It is characterized by a narrow lumen and a mucosa with longitudinal folds, covered along most of its extent by pseudostratified columnar epithelium with stereocilia. The lamina propria is rich in elastic fibers, and the thick muscular layer consists of longitudinal inner and outer layers separated by a circular layer. The abundant smooth muscle produces strong peristaltic contractions that participate in the expulsion of the spermatozoa during ejaculation.
From the epididymis the ductus (vas) deferens, a straight tube with a thick, muscular wall, continues toward the prostatic urethra and empties into it. It is characterized by a narrow lumen and a mucosa with longitudinal folds, covered along most of its extent by pseudostratified columnar epithelium with stereocilia. The lamina propria is rich in elastic fibers, and the thick muscular layer consists of longitudinal inner and outer layers separated by a circular layer. The abundant smooth muscle produces strong peristaltic contractions that participate in the expulsion of the spermatozoa during ejaculation. The ductus deferens forms part of the spermatic cord, which includes the testicular artery, the pampiniform plexus, and nerves. Before it enters the prostate, the ductus deferens dilates, forming a region called the ampulla. In this area, the epithelium becomes thicker and extensively folded. At the final portion of the ampulla, the seminal vesicles join the duct. From there on, the ductus deferens enters the prostate, opening into the prostatic urethra. The segment entering the prostate is called the ejaculatory duct. The mucous layer of the ductus deferens continues through the ampulla into the ejaculatory duct, but the muscle layer ends after the ampulla.
The ductus deferens forms part of the spermatic cord, which includes the testicular artery, the pampiniform plexus, and nerves. Before it enters the prostate, the ductus deferens dilates, forming a region called the ampulla. In this area, the epithelium becomes thicker and extensively folded. At the final portion of the ampulla, the seminal vesicles join the duct. From there on, the ductus deferens enters the prostate, opening into the prostatic urethra. The segment entering the prostate is called the ejaculatory duct. The mucous layer of the ductus deferens continues through the ampulla into the ejaculatory duct, but the muscle layer ends after the ampulla.The Seminal Vesicles
 The seminal vesicles consist of two highly tortuous tubes about 15 cm in length. When the organ is sectioned, the same tube is observed in different orientations. It has a folded mucosa that is lined with cuboidal or pseudostratified columnar epithelium rich in secretory granules. These granules have ultrastructural characteristics similar to those found in protein-synthesizing cells. The lamina propria of the seminal vesicles is rich in elastic fibers and surrounded by a thin layer of smooth muscle. The seminal vesicles are not reservoirs for spermatozoa. They are glands that produce a viscid, yellowish secretion that contains spermatozoa-activating substances such as carbohydrates, citrate, inositol, prostaglandins, and several proteins. The carbohydrates, of which fructose is the most abundant, are the source of energy for sperm motility. Seventy percent of human ejaculate originates in the seminal vesicles. The height of the epithelial cells of the seminal vesicles and the degree of activity of the secretory processes are dependent on testosterone levels.
The seminal vesicles consist of two highly tortuous tubes about 15 cm in length. When the organ is sectioned, the same tube is observed in different orientations. It has a folded mucosa that is lined with cuboidal or pseudostratified columnar epithelium rich in secretory granules. These granules have ultrastructural characteristics similar to those found in protein-synthesizing cells. The lamina propria of the seminal vesicles is rich in elastic fibers and surrounded by a thin layer of smooth muscle. The seminal vesicles are not reservoirs for spermatozoa. They are glands that produce a viscid, yellowish secretion that contains spermatozoa-activating substances such as carbohydrates, citrate, inositol, prostaglandins, and several proteins. The carbohydrates, of which fructose is the most abundant, are the source of energy for sperm motility. Seventy percent of human ejaculate originates in the seminal vesicles. The height of the epithelial cells of the seminal vesicles and the degree of activity of the secretory processes are dependent on testosterone levels. The prostate gland, the largest of the accessory glands, is pierced by the urethra and the ejaculatory ducts. The slender capsule of the gland is composed of a richly vascularized, dense irregular collagenous connective tissue interspersed with smooth muscle cells. The connective tissue stroma of the gland is derived from the capsule and is, therefore, also enriched by smooth muscle fibers in addition to their normal connective tissue cells.
The prostate gland, the largest of the accessory glands, is pierced by the urethra and the ejaculatory ducts. The slender capsule of the gland is composed of a richly vascularized, dense irregular collagenous connective tissue interspersed with smooth muscle cells. The connective tissue stroma of the gland is derived from the capsule and is, therefore, also enriched by smooth muscle fibers in addition to their normal connective tissue cells. The bulbourethral glands (Cowper's glands), 3-5 mm in diameter, are proximal to the membranous portion of the urethra and empty into it. They are tubuloalveolar glands lined with mucus-secreting simple cuboidal epithelium. Skeletal and smooth muscle cells are present in the septa that divide each gland into lobes. The secreted mucus is clear and acts as a lubricant.
The bulbourethral glands (Cowper's glands), 3-5 mm in diameter, are proximal to the membranous portion of the urethra and empty into it. They are tubuloalveolar glands lined with mucus-secreting simple cuboidal epithelium. Skeletal and smooth muscle cells are present in the septa that divide each gland into lobes. The secreted mucus is clear and acts as a lubricant. The penis is composed of three columns of erectile tissue, each enclosed by its own dense, fibrous connective tissue capsule, the tunica albuginea.
The penis is composed of three columns of erectile tissue, each enclosed by its own dense, fibrous connective tissue capsule, the tunica albuginea.- Gartner, L.P. and Hiatt, J.L. Concise Histology. 2011
- Color Textbook Histology, third edition, leslie P. Gartner
- Basic Histology, tenth edition, L. Carlos Junqueira,2003
- Histology and Cell Biology, second edition,2007
- Elsevier’s Integrated Histology,2007
Posted in 2012 , lecture , male genital system , materi kuliah
Popular Posts
-
Agranulocytosis is characterized by a greatly decreased number of circulating neutrophils. Severe neutropenia is the term usually applied to...
-
Saat ini saya sedang membimbing beberapa mahasiswa pada program Special Study di Fakultas kedokteran Universitas Udayana. Pada tahap pertam...
-
Contents : The Male Genital System The Testis Spermatogenesis Genital Ducts Genital Accessory Glands The Penis The Male ...
-
Contents : Female Reproductive System Ovaries Ovarian Follicles Uterus Menstrual Cycle Mammary Gland Female Reproductive System ...
-
Ganglia are ovoid structures containing neuronal cell bodies and glial cells supported by connective tissue . Because they serve as relay st...
-
Granulopoiesis The maturation process of granulocytes takes place with cytoplasmic changes characterized by the synthesis of a number of p...
-
The main components of the peripheral nervous system are the nerves, ganglia , and nerve endings . Nerves are bundles of nerve fibers surrou...
-
Mammalian neurons usually do not divide, and their degeneration represents a permanent loss . Peripheral nerve fibers can regenerate if thei...
-
Contractile activity in muscle cells results primarily from an interaction between two proteins: actin and myosin. Actin is present in muscl...
-
The spermatozoa are formed within the seminiferous tubules of the testes in a complex process called spermatogenesis. The continuous mainte...






Leukopoiesis Powerpoint 2012